Photo-release and luminescence:
Photocaged compounds:
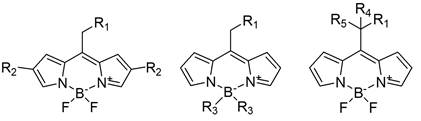
We are interested in compounds able to release photochemically a moiety of interest such as a drug. In particular, we are studying derivatives of boron-dipyrromethenes commonly known as BODIPYs. These derivatives include in their structure both, the BODIPY moiety acting as a photo-releasable protecting group and a pharmacological agent. Using light as the external stimulus to release the pharmacological agent provides spatial and temporal control over its bioactivity toward a specific target, also improving drug selectivity while decreasing adverse effects.
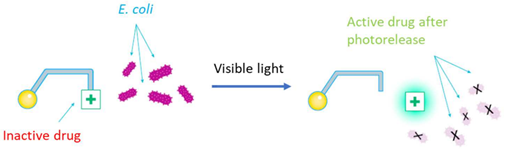
Figures adapted from DOI: 10.3390/pharmaceutics14051070
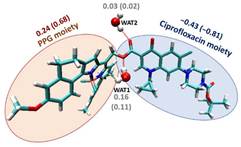
Different BODIPY derivatives have been studied for which the photo-release takes place by dissociation of an antibiotic moiety by breaking a C-O bond. Since a variety of behaviors were experimentally found for the different derivatives, we have investigated their photo-release mechanism from a computational point of view under the framework of the Time Dependent-Density Functional Theory (TD-DFT). We have concluded that after excitation to the lowest-energy singlet excited state (S1) the system reaches a minimum in this state. From there, a significantly large energy barrier in S1 along the C-O bond breaking responsible for the photo-release has been found. Hence, the photo-release in S1 is not an energetically favorable path. However, while vibrating around the S1 minimum structure, the system can populate the lower in energy triplet manifold (T1 in this case) through an InterSystem Crossing (ISC). Once in T1, the system can break the C-O bond overcoming a much lower energy barrier than the one computed in S1. Moreover, this mechanism has been computed in different environments, showing the key role of explicit water molecules for its correct description.
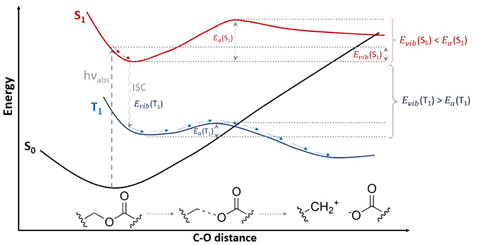
Figures adapted from DOI: 10.3390/pharmaceutics14051070
We have also studied the substitution effect on the modulation of the energy barriers found along the photo-release pathway. This point is of crucial importance for given applications, since simple chemical modifications of the BODIPY structure could lead to a significant increase in process efficiency. For this aim, we have modeled the effects induced by electron-withdrawing or -donating groups in different positions of the BODIPY skeleton.
Luminescence:
In recent years, we have investigated the mechanisms underlying bioluminescence in various systems, including fireflies and certain fungal lineages.
Regarding firefly bioluminescence, we have conducted an in-depth multi-scaling computational study, simulating the absorption and emission spectra of all potential natural chemical forms that could contribute to light emission. Additionally, we have explored the photophysical properties of designed synthetic derivatives with two primary objectives: (i) preventing protonation and keto-enol equilibria that occur in the natural molecule, (ii) tuning the emitted light to a desired wavelength.
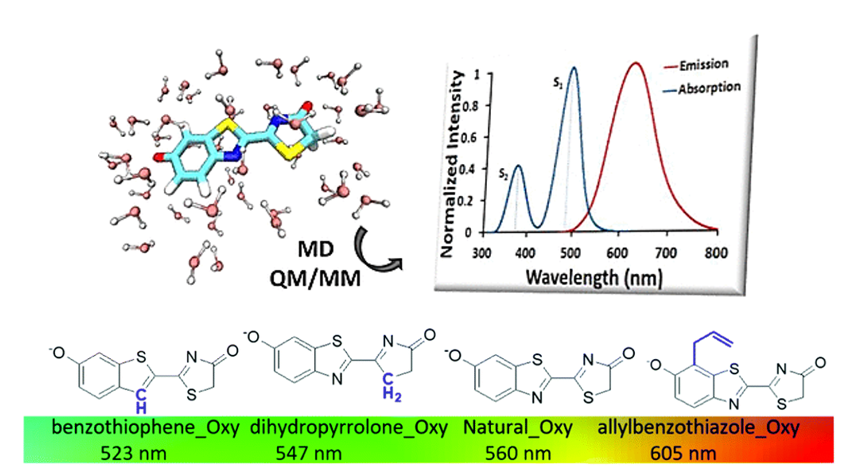
Figure adapted form DOI: 0.1039/C9CP04687A
In the case of fungal bioluminescence, we have carried out a combined experimental and computational analysis to identify the main chemical species responsible for light emission and to assess the impact of pH on this process. Furthermore, using state-of-the-art multiconfigurational quantum chemistry, we have mapped the complete mechanistic pathway from the endoperoxide intermediate to the emitting species, evaluating the feasibility of different routes. Specifically, we found that thermal CO₂ detachment followed by excited-state peroxide opening (thermal-chemiluminescence) can compete with an alternative pathway in which the endoperoxide first undergoes excited-state opening, followed by CO₂ detachment in the same excited state (excited-state chemiluminescence). Differences in energy requirements, as well as the potential for direct population of the emitting species from the intersection seam between ground and excited states, support a kinetically favorable thermal-chemiluminescent pathway. This work provides, for the first time, a detailed mechanistic description of fungal bioluminescence.
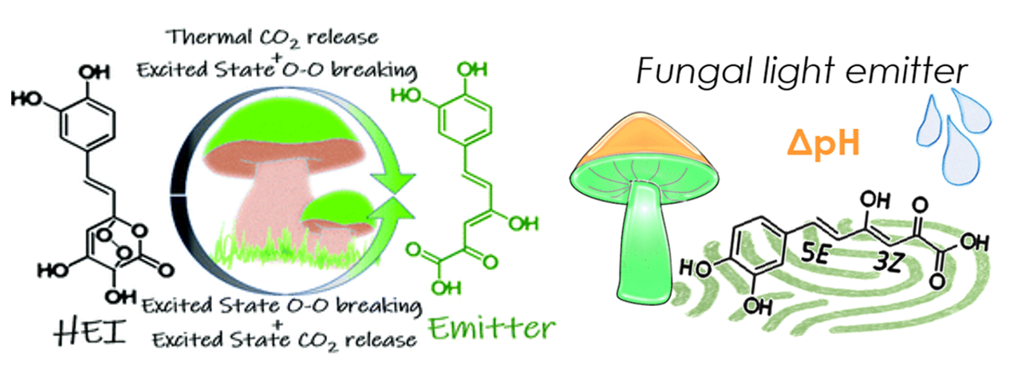
Figures adapted from DOI: 10.1039/D0CP05037G and 10.1021/acs.joc.0c00246